
HL Paper 2
Enthalpy changes depend on the number and type of bonds broken and formed.
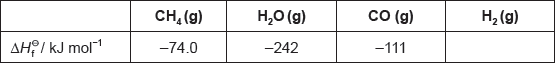
The table lists the standard enthalpies of formation, \(\Delta H_{\text{f}}^\Theta \), for some of the species in the reaction above.
Enthalpy changes depend on the number and type of bonds broken and formed.
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
Outline why no value is listed for H2(g).
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
The table lists standard entropy, SΘ, values.
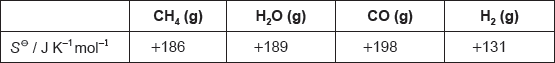
Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
Determine the temperature, in K, above which the reaction becomes spontaneous.
Markscheme
bonds broken: 4(C–H) + 2(H–O)/4(414) + 2(463)/2582 «kJ»
bonds made: 3(H–H) + C≡O/3(436) + 1077/2385 «kJ»
ΔH «= ΣBE(bonds broken) – ΣBE(bonds made) = 2582 – 2385» = «+» 197 «kJ»
Award [3] for correct final answer.
Award [2 max] for –197 «kJ».
[3 marks]
\(\Delta H_{\text{f}}^\Theta \) for any element = 0 «by definition»
OR
no energy required to form an element «in its stable form» from itself
[1 mark]
ΔHΘ « = \(\sum {\Delta H_{\text{f}}^\Theta } \)(products) – \(\sum {\Delta H_{\text{f}}^\Theta } \)(reactants) = –111 + 0 – [–74.0 + (–242)]»
= «+» 205 «kJ»
[1 mark]
«ΔSΘ = ΣSΘproducts – ΣSΘreactants = 198 + 3 × 131 – (186 + 189) =» «+» 216 «J K–1»
[1 mark]
«ΔGΘ = ΔHΘ – TΔSΘ = 205 kJ – 298 K × \(\frac{{216}}{{1000}}\) kJ K–1 =» «+» 141 «kJ»
[1 mark]
«ΔHΘ = TΔSΘ»
«\({\text{T}} = \frac{{\Delta {H^\Theta }}}{{\Delta {S^\Theta }}} = \frac{{205000{\text{ J}}}}{{216{\text{ J }}{{\text{K}}^{ - 1}}}}\)»
«T =» 949 «K»
Do not award a mark for negative value of T.
[1 mark]
Examiners report
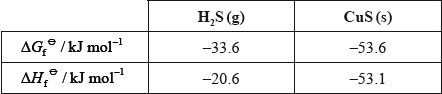
Consider the reaction:
\[{\text{CuS(s)}} + {{\text{H}}_2}{\text{(g)}} \to {\text{Cu(s)}} + {{\text{H}}_2}{\text{S(g)}}\]
Given:
Deduce and explain the sign of the entropy change for the following reaction.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\]
Suggest why the \(\Delta H_{\text{f}}^\Theta \) values for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) and Cu(s) are not given in the table.
Determine the standard enthalpy change at 298 K for the reaction.
Determine the standard free energy change at 298 K for the reaction. Deduce whether or not the reaction is spontaneous at this temperature.
Determine the standard entropy change at 298 K for the reaction.
Estimate the temperature, in K, at which the standard change in free energy equals zero. You should assume that the values of the standard enthalpy and entropy changes are not affected by the change in temperature.
Markscheme
negative;
liquid more ordered than gaseous phase or vice-versa / OWTTE;
\(\Delta H_{\text{f}}^\Theta \) of an element (in its most stable state) is zero (since formation of an element from itself is not a reaction) / OWTTE;
Do not allow an answer such as because they are elements.
\(\Delta {H^\Theta }( = (1)( - 20.6) - (1)( - 53.1)) = 32.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}/32500{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Allow 32.5 (kJ) or 3.25 \( \times \) 104 (J).
\(\Delta {G^\Theta }( = (1)( - 33.6) - (1)( - 53.6)) = 20.0{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}/20000{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Allow 20.0 (kJ) or 2.00 \( \times \) 104 (J).
non-spontaneous;
\(\Delta {S^\Theta }( = (\Delta {H^\Theta } - \Delta {G^\Theta })/T = (32.5 - 20.0)(1000)/298) = 41.9{\text{ (J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}/\)
\(4.19 \times {10^{ - 2}}{\text{ (kJ}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Allow 41.9 (J\(\,\)K–1) or 4.19 \( \times \) 10–2 (kJ\(\,\)K–1).
\(T{\text{ }}\left( { = \Delta H/\Delta S = (32.5 \times 1000)/(41.9)} \right) = 776{\text{ (K)}}\);
Examiners report
The negative nature of the change gained a mark, but the explanations sometimes lacked clarity and states often were not referred to.
In (i), often there was no mention of element.
(ii) to (iv) was often very well done, though as usual some candidates struggled with units.
(ii) to (iv) was often very well done, though as usual some candidates struggled with units.
(ii) to (iv) was often very well done, though as usual some candidates struggled with units.
An example of a homogeneous reversible reaction is the reaction between hydrogen and iodine.
\[{{\text{H}}_2}{\text{(g)}} + {{\text{I}}_2}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}\]
Propene can be hydrogenated in the presence of a nickel catalyst to form propane. Use the data below to answer the questions that follow.

At a temperature just above 700 K it is found that when 1.60 mol of hydrogen and 1.00 mol of iodine are allowed to reach equilibrium in a \({\text{4.00 d}}{{\text{m}}^{\text{3}}}\) flask, the amount of hydrogen iodide formed in the equilibrium mixture is 1.80 mol. Determine the value of the equilibrium constant at this temperature.
Outline why the value for the standard enthalpy change of formation of hydrogen is zero.
Calculate the standard enthalpy change for the hydrogenation of propene.
Calculate the standard entropy change for the hydrogenation of propene.
Determine the value of \(\Delta {G^\Theta }\) for the hydrogenation of propene at 298 K.
At 298 K the hydrogenation of propene is a spontaneous process. Determine the temperature above which propane will spontaneously decompose into propene and hydrogen.
Markscheme
amount of \({{\text{H}}_2}\) remaining at equilibrium \( = 1.60 - \frac{{1.80}}{2} = 0.70{\text{ mol}}\);
amount of \({{\text{I}}_2}\) remaining at equilibrium \( = 1.0 - \frac{{1.80}}{2} = 0.10{\text{ mol}}\);
\({K_{\text{c}}} = \frac{{{{(1.80/4.0)}^2}}}{{(0.70/4.00) \times (0.10/4.00)}}/\frac{{{{1.80}^2}}}{{0.70 \times 0.10}}\);
\({K_{\text{c}}} = \frac{{{{(1.80)}^2}}}{{0.70 \times 0.10}} = 46.3\);
Award [4] for correct final answer.
by definition \(\Delta H_{\text{f}}^\Theta \) of elements (in their standard states) is zero / no reaction involved / OWTTE;
\(\Delta H = - 104 - ( + 20.4)\);
\( = - 124.4{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [1 max] for 124.4 (kJ\(\,\)mol−1).
Award [2] for correct final answer.
\(\Delta S = 270 - (267 + 131)\);
\( = - 128{\text{ (J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [1 max] for +128 ( J\(\,\)K−1mol−1).
Award [2] for correct final answer.
\(\Delta G = \Delta H - {\text{T}}\Delta S = - 124.4 - \frac{{( - 128 \times 298)}}{{1000}}\);
\( = - 86.3{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Units needed for the mark.
Award [2] for correct final answer.
Allow ECF if only one error in first marking point.
\(\Delta G = \Delta H - {\text{T}}\Delta S = 0/\Delta H = {\text{T}}\Delta S\);
\({\text{T}} = \frac{{ - 124.4}}{{ - 128/1000}} = 972{\text{ K}}/699{\text{ }}^\circ {\text{C}}\);
Only penalize incorrect units for T and inconsistent ΔS value once in (iv) and (v).
Examiners report
This was the most popularly answered question. Most candidates were able to give a good description of the characteristics of homogenous equilibrium, and apply Le Chatelier‟s Principle to explain the effect of catalysts and changes of temperature and pressure on the position of equilibrium and the equilibrium constant. A good majority were able to calculate the value of \({K_{\text{c}}}\) although a significant number of candidates incorrectly used the initial rather than the equilibrium concentrations.
Although most candidates clearly understood the concept of standard enthalpy change of formation many were unable to explain why the value for hydrogen is zero. Many responses neglected to mention that \({{\text{H}}_{\text{2}}}\) is an element in its standard state.
Most candidate were able to calculate \(\Delta H\) and \(\Delta S\) although some inverted the equation and gave a positive value instead of negative answer or confused the values for propane and propene.
There were some inconsistencies in the use of units and significant figures when calculating \(\Delta G\) from \(\Delta H\) and \(\Delta S\) values although there was a significant improvement in this area compared to previous.
There were some inconsistencies in the use of units and significant figures when calculating \(\Delta G\) from \(\Delta H\) and \(\Delta S\) values although there was a significant improvement in this area compared to previous.
There were some inconsistencies in the use of units and significant figures when calculating \(\Delta G\) from \(\Delta H\) and \(\Delta S\) values although there was a significant improvement in this area compared to previous. This error resulted in some very strange temperatures for the thermal decomposition of propane to propene.
The photochemical chlorination of methane can occur at low temperature.
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
Markscheme
«ΔH θ =» –82.0 «kJ» –92.3 «kJ» – (–74.0 «kJ»)
«ΔH θ =» –100.3 «kJ»
Award [2] for correct final answer.
[2 marks]
Examiners report
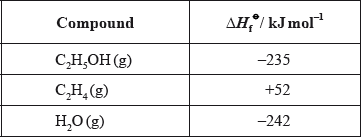
The reaction between ethene and steam is used in the industrial production of ethanol.
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{H}}_{\text{2}}}{\text{O(g)}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH(g)}}\]
The enthalpy change of the reaction can be calculated either by using average bond enthalpies or by using standard enthalpies of formation.
Determine the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the average bond enthalpies in Table 10 of the Data Booklet.
(i) Define the term standard enthalpy change of formation.
(ii) Determine the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), between ethene and steam using the enthalpy change of formation values given below.
Comment on which of the values obtained in (a) and (b)(ii) is more accurate, giving a reason.
Predict the sign of the entropy change of the reaction, \(\Delta S\), giving a reason.
Markscheme
(bonds broken) C=C and O–H / 612 + 464 / 1076;
(bonds formed) C–C and C–H and C–O / 347 + 413 + 358 / 1118;
OR
(bonds broken) C=C and two O–H and four C–H / 612 + 4(413) + 2(464) / 3192;
(bonds formed) C–C and five C–H and C–O and O–H / 347 + 5(413) + 358 + 464 / 3234;
Ignore signs (+ and –) in M1 and M2. These two marks are awarded for recognizing the correct bonds.
enthalpy change \( = - 42{\text{ (kJ)}}\);
Correct sign is necessary for awarding M3.
Award [3] for the correct final answer.
Do not penalize candidates using the former Data Booklet bond energy values (348, 412 and 463) (final answer will then be –45(kJ)).
(i) heat/enthalpy change when 1 mol of a compound/substance is formed;
from its elements in their standard states/at \({\text{100 kPa/1}}{{\text{0}}^{\text{5}}}{\text{ Pa}}\);
Allow 1.01 \( \times \) 105 Pa/101 kPa/1 atm as an alternative to 100 kPa/105 Pa.
Allow under standard conditions or standard ambient temperature and pressure as an alternative to 100 kPa/105 Pa.
Allow “energy needed/absorbed” as an alternative to “heat/enthalpy change”.
Temperature is not required in definition, allow if quoted (eg, 298 K / 25 °C).
(ii) \(( - 235) - (52 - 242)/\Delta H = \Sigma \Delta H_{\text{f}}^\Theta {\text{(products)}} - \Sigma \Delta H_{\text{f}}^\Theta {\text{(reactants)}}\);
–45 (kJ);
Award [2] for the correct final answer.
Award [1] for +45 or 45.
value in (b)(ii) (is more accurate) as values used in (a) are average values / value in (b)(ii) (is more accurate) as exact bond enthalpy depends on the surroundings of the bond / OWTTE;
negative and fewer number of moles/molecules (of gas);
Examiners report
More than half of the candidates identified the correct types and numbers of bonds, and many calculated the enthalpy change of reaction correctly gaining full marks. Common mistakes included reversing the signs of bonds broken and bonds formed, and using incorrect types or numbers of bonds, and arithmetic errors.
(i) Less than half of the candidates answered the question correctly. Some were not specific in the definition of the standard enthalpy change of formation, while others had totally incorrect answers such as the formation of the compound from gaseous atoms.
(ii) The majority of candidates calculated the enthalpy change correctly. Some candidates made arithmetic errors.
More than half of the candidates referred to bond enthalpies being average values that lead to a less accurate calculated value of the enthalpy change.
To determine the enthalpy change of combustion of methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\), 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water from 24.5 °C to 45.8 °C.
Methanol can be produced according to the following equation.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_3}{\text{OH(l)}}\]
The manufacture of gaseous methanol from CO and \({{\text{H}}_{\text{2}}}\) involves an equilibrium reaction.
\({\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{OH(g)}}\) \(\Delta {H^\Theta } < 0\)
Calculate the standard enthalpy change of this reaction, using the values of enthalpy of combustion in Table 12 of the Data Booklet.
Calculate the standard entropy change for this reaction, \(\Delta {S^\Theta }\), using Table 11 of the Data Booklet and given:
\({S^\Theta }{\text{(CO)}} = 198{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{ and }}{S^\Theta }{\text{(}}{{\text{H}}_{\text{2}}}{\text{)}} = 131{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate, stating units, the standard free energy change for this reaction, \(\Delta {G^\Theta }\), at 298 K.
Predict, with a reason, the effect of an increase in temperature on the spontaneity of this reaction.
1.00 mol of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\) is placed in a closed container of volume \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) until equilibrium is reached with CO and \({{\text{H}}_{\text{2}}}\). At equilibrium 0.492 mol of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\) are present. Calculate \({K_{\text{c}}}\).
Markscheme
\({\text{C}}{{\text{H}}_3}{\text{OH}} + \frac{3}{2}{{\text{O}}_2} \to {\text{C}}{{\text{O}}_2} + 2{{\text{H}}_2}{\text{O}}\) \(\Delta H_{\text{c}}^{^\Theta } = - 726{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\)
\({\text{CO}} + \frac{1}{2}{{\text{O}}_2} \to {\text{C}}{{\text{O}}_2}\) \(\Delta H_{\text{c}}^{^\Theta } = - 283{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\)
\({{\text{H}}_2} + \frac{1}{2}{{\text{O}}_2} \to {{\text{H}}_2}{\text{O}}\) \(\Delta H_{\text{c}}^{^\Theta } = - 286{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\)
Award [1 max] for three correct values.
Mark can be implicit in calculations.
\((\Delta H_{\text{R}}^{^\Theta } = ){\text{ }}2( - 286) + ( - 283) - ( - 726)\);
\( - {\text{129 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2 max] for +129 (kJ\(\,\)mol–1).
\((\Delta {S^\Theta } = 240 - 198 - 2 \times 131 = ){\text{ }} - 220{\text{ (J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\left( { - 129 - 298( - 0.220) = } \right){\text{ }} - 63.4{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Award [1] for correct numerical answer and [1] for correct unit if the conversion has been made from J to kJ for \(\Delta {S^\Theta }\).
not spontaneous at high temperature;
\(T\Delta {S^\Theta } < \Delta {H^\Theta }\) and \(\Delta {G^\Theta }\) positive;
\(n{\text{(CO)}} = 0.508{\text{ (mol)}}\);
\(n({{\text{H}}_2}) = 2 \times 0.508{\text{ (mol)}}\);
\({K_{\text{c}}}{\text{ }}\left( { = \frac{{0.492}}{{0.508 \times {{(2 \times 0.508)}^2}}}} \right) = 0.938\);
Accept answer in range between 0.930 and 0.940.
Award [3] for correct final answer.
Award [2] for Kc = 1.066 if (c)(ii) is correct.
Examiners report
In (i), the most common error was \( + {\text{129 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) but in (ii) the answer was often correct.
In (i), the most common error was \( + {\text{129 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) but in (ii) the answer was often correct.
Units tended to get muddled in (iii) and many marks were awarded as “error carried forward”.
Few were able to explain the \(\Delta H\) and \(T\Delta S\) relationship in detail in (iv).
Equilibrium was well understood in general with many candidates gaining one of the two available marks. “Equal rates” was more often given than the constancy of macroscopic properties for the second mark. The \({K_{\text{c}}}\) expression was given correctly by the vast majority of candidates (including the correct brackets and indices) but many had difficulty with the equilibrium concentrations in (iii).
The changes in equilibrium position were well understood for the most part although if a mark were to be lost it was for not mentioning the number of moles of gas.
Two chemistry students wished to determine the enthalpy of hydration of anhydrous magnesium sulfate. They measured the initial and the highest temperature reached when anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}{\text{(s)}}\), was dissolved in water. They presented their results in the table below.

The students repeated the experiment using 6.16 g of solid hydrated magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}} \bullet {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(s)}}\), and \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. They found the enthalpy change, \(\Delta {H_2}\) , to be \( + {\text{18 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
The enthalpy of hydration of solid anhydrous magnesium sulfate is difficult to determine experimentally, but can be determined using the diagram below.
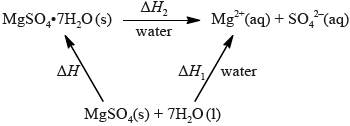
(i) Calculate the amount, in mol, of anhydrous magnesium sulfate.
(ii) Calculate the enthalpy change, \(\Delta {H_1}\), for anhydrous magnesium sulfate dissolving in water, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). State your answer to the correct number of significant figures.
(i) Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the hydration of solid anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}\).
(ii) The literature value for the enthalpy of hydration of anhydrous magnesium sulfate is \( - 103{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage difference between the literature value and the value determined from experimental results, giving your answer to one decimal place. (If you did not obtain an answer for the experimental value in (b)(i) then use the value of \( - 100{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Another group of students experimentally determined an enthalpy of hydration of \( - 95{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Outline two reasons which may explain the variation between the experimental and literature values.
Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic limestone. This limestone is a mixture of magnesium carbonate and calcium carbonate.
(i) State the equation for the reaction of sulfuric acid with magnesium carbonate.
(ii) Deduce the Lewis (electron dot) structure of the carbonate ion, giving the shape and the oxygen-carbon-oxygen bond angle.
Lewis (electron dot) structure:
Shape:
Bond angle:
(iii) There are three possible Lewis structures that can be drawn for the carbonate ion, which lead to a resonance structure. Explain, with reference to the electrons, why all carbon-oxygen bonds have the same length.
(iv) Deduce the hybridization of the carbon atom in the carbonate ion.
Markscheme
(i) \(n{\text{(MgS}}{{\text{O}}_4}{\text{)}} = \left( {\frac{{3.01}}{{120.37}} = } \right)0.0250{\text{ (mol)}}\);
(ii) energy released \( = 50.0 \times 4.18 \times 9.7 \times 2027{\text{ (J)}}/2.027{\text{ (kJ)}}\);
\(\Delta {H_1} = - 81{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [2] for correct answer.
Award [2] if 53.01 is used giving an answer of –86 (kJ mol–1).
Award [1 max] for +81/81/+86/86 (kJ mol−1).
Award [1 max] for –81000/–86000 if units are stated as J mol−1.
Allow answers to 3 significant figures.
(i) \(\Delta H{\text{ (}} = \Delta {H_1} - \Delta {H_2}{\text{)}} = - 99{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [1] if –86 is used giving an answer of –104 (kJ mol–1).
(ii) \(\frac{{(103 - 99)}}{{103}} \times 100 = 3.9\% \);
Accept answer of 2.9% if –100 used but only if a value for (b)(i) is not present.
Award [1] if –104 is used giving an answer of 1.0% .
Accept correct answers which are not to 1 decimal place.
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) not completely anhydrous / OWTTE;
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) is impure;
heat loss to the atmosphere/surroundings;
specific heat capacity of solution is taken as that of pure water;
experiment was done once only so it is not scientific;
density of solution is taken to be \({\text{1 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\);
mass of \({\text{7}}{{\text{H}}_2}{\text{O}}\) ignored in calculation;
uncertainty of thermometer is high so temperature change is unreliable;
literature values determined under standard conditions, but this experiment is not;
all solid not dissolved;
(i) \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{MgC}}{{\text{O}}_3}{\text{(s)}} \to {\text{MgS}}{{\text{O}}_4}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
Do not accept H2CO3.
(ii) 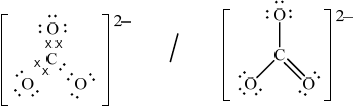 ;
;
Accept crosses, lines or dots as electron pairs.
Accept any correct resonance structure.
Award [0] if structure is drawn without brackets and charge.
Award [0] if lone pairs not shown on O atoms.
shape: trigonal/triangular planar;
bond angle: 120° ;
Accept answers trigonal/triangular planar and 120° if M1 incorrect, but no other answers should be given credit.
(iii) (pi/\(\pi \)) electrons are delocalized/spread over more than two nuclei / charge spread (equally) over all three oxygens;
(iv) \({\text{s}}{{\text{p}}^{\text{2}}}\);
Examiners report
The use of 3.01 for the mass in the expression in \(Q = mc\Delta T\) was common, candidates were able to score in the subsequent parts and many did so, although there was often a confusion between the value Q and the required answer for \(\Delta H\). In part c) most candidates understood the error due to heat loss, but few scored the second mark, usually quoting an answer involving an error generally that was far too vague. The inability to construct a balanced equation was disappointing, many lost credit for giving \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) as a product. The score for the structure of the carbonate ion was often lost due to the failure to show that a charge is present on the ion, however, the shape and bond angle were known well, as was delocalisation and hybridisation.
The use of 3.01 for the mass in the expression in \(Q = mc\Delta T\) was common, candidates were able to score in the subsequent parts and many did so, although there was often a confusion between the value Q and the required answer for \(\Delta H\). In part c) most candidates understood the error due to heat loss, but few scored the second mark, usually quoting an answer involving an error generally that was far too vague. The inability to construct a balanced equation was disappointing, many lost credit for giving \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) as a product. The score for the structure of the carbonate ion was often lost due to the failure to show that a charge is present on the ion, however, the shape and bond angle were known well, as was delocalisation and hybridisation.
The use of 3.01 for the mass in the expression in \(Q = mc\Delta T\) was common, candidates were able to score in the subsequent parts and many did so, although there was often a confusion between the value Q and the required answer for \(\Delta H\). In part c) most candidates understood the error due to heat loss, but few scored the second mark, usually quoting an answer involving an error generally that was far too vague. The inability to construct a balanced equation was disappointing, many lost credit for giving \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) as a product. The score for the structure of the carbonate ion was often lost due to the failure to show that a charge is present on the ion, however, the shape and bond angle were known well, as was delocalisation and hybridisation.
The use of 3.01 for the mass in the expression in \(Q = mc\Delta T\) was common, candidates were able to score in the subsequent parts and many did so, although there was often a confusion between the value Q and the required answer for \(\Delta H\). In part c) most candidates understood the error due to heat loss, but few scored the second mark, usually quoting an answer involving an error generally that was far too vague. The inability to construct a balanced equation was disappointing, many lost credit for giving \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) as a product. The score for the structure of the carbonate ion was often lost due to the failure to show that a charge is present on the ion, however, the shape and bond angle were known well, as was delocalisation and hybridisation.
One important property of a rocket fuel mixture is the large volume of gaseous products formed which provide thrust. Hydrazine, N2H4, is often used as a rocket fuel. The combustion of hydrazine is represented by the equation below.
\[\begin{array}{*{20}{l}} {{{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}({\text{g)}} \to {{\text{N}}_{\text{2}}}({\text{g)}} + 2{{\text{H}}_{\text{2}}}{\text{O(g)}}}&{\Delta H_{\text{c}}^\Theta = - {\text{585 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Comment on the environmental safety of the products of the reaction of \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) with \({{\text{O}}_{\text{2}}}\) and the reaction of \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) with \({{\text{F}}_{\text{2}}}\).
Markscheme
(\({{\text{N}}_{\text{2}}}\) inert) HF (weak) acid compared to \({{\text{H}}_2}{\text{O}}\) / HF toxic / products of reactions of HF with environment/soil are harmful to environment / OWTTE;
Examiners report
A matter of serious concern is the number of candidates who identified nitrogen as harmful to the environment or a greenhouse gas.
Hydrazine, N2H4, is a valuable rocket fuel.
The equation for the reaction between hydrazine and oxygen is given below.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{l)}} + {{\text{O}}_2}({\text{g)}} \to {{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(l)}}\]
The reaction between \({{\text{N}}_2}{{\text{H}}_4}({\text{aq)}}\) and \({\text{HCl(aq)}}\) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Draw the Lewis (electron dot) structure for N2H4 showing all valence electrons.
(ii) State and explain the H–N–H bond angle in hydrazine.
Hydrazine and ethene, C2H4, are hydrides of adjacent elements in the periodic table. The boiling point of hydrazine is much higher than that of ethene. Explain this difference in terms of the intermolecular forces in each compound.
(i) The enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of liquid hydrazine is \({\text{50.6 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Use this value, together with data from Table 12 of the Data Booklet, to calculate the enthalpy change for this reaction.
(ii) Use the bond enthalpy values from Table 10 of the Data Booklet to determine the enthalpy change for this reaction.
(iii) Identify the calculation that produces the most accurate value for the enthalpy change for the reaction given and explain your choice.
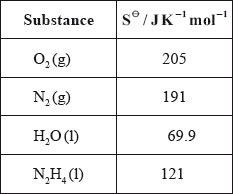
(iv) Calculate \(\Delta {S^\Theta }\) for the reaction using the data below and comment on its magnitude.
(v) Calculate \(\Delta {G^\Theta }\) for the reaction at 298 K.
(vi) Predict, giving a reason, the spontaneity of the reaction above at both high and low temperatures.
The reaction between \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}}\) and HCl(aq) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Identify the type of reaction that occurs.
(ii) Predict the value of the H–N–H bond angle in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
(iii) Suggest the type of hybridization shown by the nitrogen atoms in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
Markscheme
(i) 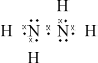 ;
;
Accept x’s, dots or lines for electron pairs
(ii) H–N–H \( < \) 109 / any angle between 104° and 109°;
due to four centres of electron charge / four electron pairs (one of which is a lone \({{\text{e}}^ - }\) pair);
extra repulsion due to lone electron pairs;
Do not allow ECF for wrong Lewis structures.
weaker van der Waals’/London/dispersion/intermolecular forces in ethene;
stronger (intermolecular) hydrogen bonding in hydrazine;
If no comparison between strengths then [1 max].
(i) \(\Delta H_{\text{r}}^\Theta = \Sigma \Delta H_{\text{f}}^\Theta {\text{ products}} - \Sigma \Delta H_{\text{f}}^\Theta {\text{ reactants}}\);
Can be implied by working.
\(\Delta H_{\text{f}}^\Theta {\text{(}}{{\text{H}}_2}{\text{O(l))}} = - 286{\text{ (kJ)}}\);
\(\Delta H_{\text{r}}^\Theta = 2( - 286) - 50.6 = - 622.6{\text{ (kJ)}}\);
(ii) bonds broken: 4N–H, N–N, O=O / \( + {\text{2220 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
bonds formed: N\(\equiv\)N, 4O-H / \( - 2801{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\( - 581{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
(iii) value based on \(\Delta {H_{\text{f}}}\) more accurate;
\(\Delta {H_{\text{f}}}\) accurate for compounds in reaction;
bond energy calculation assumes average bond energies;
(bond energy calculation) only applies to gaseous states / ignores intermolecular bonds;
(iv) \(\Delta {S^\Theta } = \Sigma {S^\Theta }{\text{ (products)}} - \Sigma {S^\Theta }{\text{ (reactants)}}\);
Can be implied by working.
\( = 191 + (2 \times 69.9) - 205 - 121 = + 4.8{\text{ (J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
small value since number of mol of g on both sides the same;
(v) \(\Delta {G^\Theta } = - 622.6 - 298(0.0048)\);
\( = - 624.0{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Allow 623.9 to 624.1.
(vi) all reactions are spontaneous;
\(\Delta G\) is negative (at high temperatures and low temperatures);
(i) acid-base/neutralization;
(ii) 109°/109.5°;
(iii) sp3;
No ECF if bond angle incorrect in (ii).
Examiners report
The Lewis structure for hydrazine proved to be difficult for some in (a). Incorrect answers had double bonds appearing between the two nitrogen atoms or lone pairs missing. Those who could draw the correct structure in (i) gave the correct bond angle, but the explanation was often incomplete. Few mentioned either the four electron domains around the central atom or the extra repulsion of the lone pair.
In part (b) most candidates knew that hydrogen bonding was present in hydrazine and Van der Waals‟ forces in ethene but failed to give a comparison of the relative strength of the intermolecular forces.
Some candidates struggled to calculate the enthalpy changes from enthalpy changes of formation in (c) (i) as they were unable to relate the enthalpy change of combustion of hydrogen to the enthalpy change of formation of water.
The bond energy and entropy calculations were more successful with many candidates benefitting from ECF from their incorrect Lewis structures in (a). It was encouraging to see many correct unit conversions for the calculation of \(\Delta G\). A number of candidates incorrectly described the combination of hydrazine and hydrochloric acid as a redox reaction, but many were able to identify the bond angle and hybridization in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
Phosphoryl chloride, \({\text{POC}}{{\text{l}}_{\text{3}}}\), is a dehydrating agent.
\({\text{POC}}{{\text{l}}_{\text{3}}}\left( {\text{g}} \right)\) decomposes according to the following equation.
\[{\text{2POC}}{{\text{l}}_3}{\text{(g)}} \to {\text{2PC}}{{\text{l}}_3}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}}\]
POCl3 can be prepared by the reaction of phosphorus pentachloride, PCl5 , with tetraphosphorus decaoxide, P4O10.
PCl3 and Cl– can act as ligands in transition metal complexes such as Ni(PCl3)4 and [Cr(H2O)3Cl3].
Predict and explain the sign of the entropy change, \(\Delta S\), for this reaction.
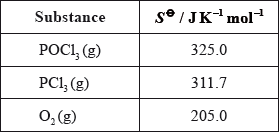
Calculate the standard entropy change for the reaction, \(\Delta {S^\Theta }\), in \({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.
Define the term standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \).
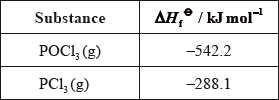
Calculate the standard enthalpy change for the reaction, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.
Determine the standard free energy change for the reaction, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), at 298 K.
Deduce the temperature, in K, at which the reaction becomes spontaneous.
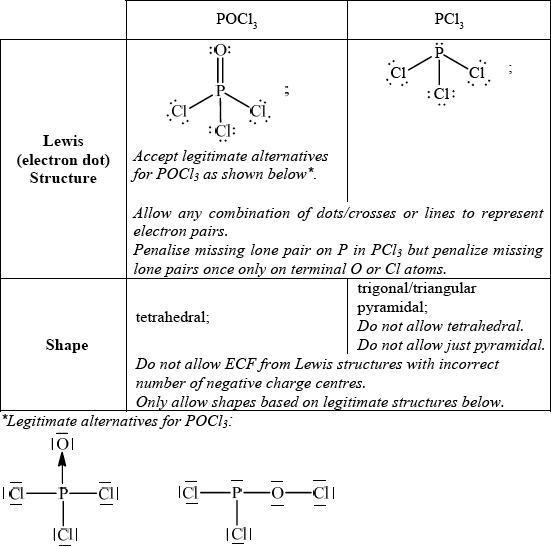
Deduce the Lewis (electron dot) structure of POCl3 (with P as the central element) and PCl3 and predict the shape of each molecule, using the valence shell electron pair repulsion theory (VSEPR).
State and explain the Cl–P–Cl bond angle in PCl3.
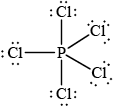
Deduce the Lewis (electron dot) structure of PCl5.
Predict the shape of this molecule, using the valence shell electron pair repulsion theory (VSEPR).
Identify all the different bond angles in PCl5.
PCl3Br2 has the same molecular shape as PCl5. Draw the three isomers of PCl3Br2 and deduce whether each isomer is polar or non-polar.
Define the term ligand.
Explain why the complex [Cr(H2O)3Cl3] is coloured.
Markscheme
2 mol (g) going to 3 mol (g)/increase in number of particles, therefore entropy increases/\(\Delta S\) positive / OWTTE;
Accept if numbers of moles of gas are given below the equation.
\(\left( {\Delta {S^\Theta } = [(2)(311.7) + (205.0)] - (2)(325.0) = } \right)( + )178.4{\text{ }}({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}})\);
heat/enthalpy change/required/absorbed when 1 mol of a compound is formed from its elements in their standard states/at 100 kPa/105 Pa/1 bar;
Allow 1.01 \( \times \) 105 Pa/101 kPa/1 atm.
Allow under standard conditions or standard temperature and pressure.
Temperatures not required in definition, allow if quoted (for example, 298 K/ 25 °C – most common) but pressure value must be correct if stated.
\(\left( {\Delta {H^\Theta } = [(2)( - 288.1)] - [(2)( - 542.2)]) = } \right)( + )508.2{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
\(\left( {\Delta {G^\Theta } = \Delta {H^\Theta } - T\Delta {S^\Theta } = (508.2) - (298)\left( {\frac{{178.4}}{{1000}}} \right) = } \right){\text{ ( + )}}455.0{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
\(T > \left( {\frac{{\Delta {H^\Theta }}}{{\Delta {S^\Theta }}} = \frac{{508.2}}{{\left( {\frac{{178.4}}{{1000}}} \right)}} = } \right){\text{ }}2849{\text{ (K)}}/2576\) (°C);
Allow temperatures in the range 2848–2855 K.
Accept T = 2849(K) .
No ECF for temperatures T in the range 0–100 K.
allow any bond angle in the range 100° to less than 109° (experimental value is100°);
due to four negative charge centres/four electron pairs/four electron domains (one of which is a lone pair)/tetrahedral arrangement of electron pairs/domains;
extra repulsion due to lone pair electrons / lone pairs occupy more space (than bonding pairs) so Cl–P–Cl bond angle decreases from 109.5° / OWTTE;
 ;
;
Allow any combination of dots/crosses or lines to represent electron pairs.
Do not penalise missing lone pairs on Cl if already penalised in (b)(i).
trigonal/triangular bipyramidal;
Do not allow ECF from Lewis structures with incorrect number of negative charge centres.
120° and 90°/180°;
Ignore other bond angles such as 240° and 360°.
Apply list principle if some correct and incorrect angles given.
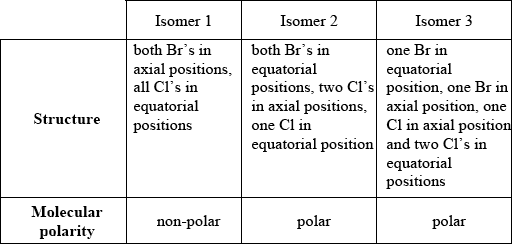
Award [1] for correct structure and molecular polarity.
Award [1 max] for correct representations of all three isomers.
Lone pairs not required.
species with lone/non-bonding pair (of electrons);
which bonds to metal ion (in complex) / which forms dative (covalent)/coordinate bond to metal ion (in complex);
unpaired electrons in d orbitals / d sub-level partially occupied;
d orbitals split (into two sets of different energies);
frequencies of (visible) light absorbed by electrons moving from lower to higher d levels;
colour due to remaining frequencies / complementary colour transmitted;
Allow wavelength as well as frequency.
Do not accept colour emitted.
Examiners report
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
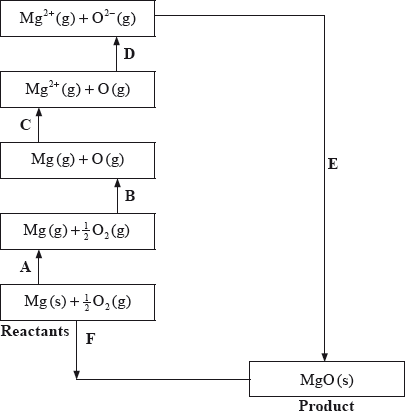
The Born-Haber cycle for MgO under standard conditions is shown below.
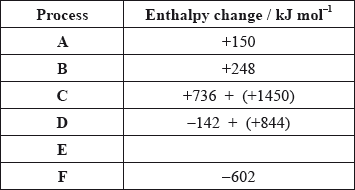
The values are shown in the table below.
Identify the processes represented by A, B and D in the cycle.
Define the enthalpy change, F.
Determine the value of the enthalpy change, E.
Define the enthalpy change C for the first value. Explain why the second value is significantly larger than the first.
The inter-ionic distance between the ions in NaF is very similar to that between the ions in MgO. Suggest with a reason, which compound has the higher lattice enthalpy value.
The standard enthalpy change of three combustion reactions is given below in kJ.
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{7}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{4C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 3120} \\ {{\text{2}}{{\text{H}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - {\text{572}}} \\ {{{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + 2{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - {\text{1411}}} \end{array}\]
Based on the above information, calculate the standard change in enthalpy, \(\Delta {H^\Theta }\), for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Predict, stating a reason, whether the sign of \({\Delta {S^\Theta }}\) for the above reaction would be positive or negative.
Discuss why the above reaction is non-spontaneous at low temperature but becomes spontaneous at high temperatures.
Using bond enthalpy values, calculate \(\Delta {H^\Theta }\) for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Suggest with a reason, why the values obtained in parts (b) (i) and (b) (iv) are different.
Markscheme
A: sublimation/atomization;
B: atomization/half dissociation enthalpy;
D: (sum of 1st and 2nd) electron affinity;
Do not accept vaporization for A and B.
Accept \(\Delta {H_{AT}}\)\( \cdot \) or \(\Delta {H_{EA}}\).
enthalpy change when one mole of the compound is formed from its elements (in their standard states);
under standard conditions / 25 °C/298 K and 1 atm/\({\text{101.3 kPa/1.01}} \times {\text{105 Pa}}\);
\( - 602 = 150 + 248 + 2186 + 702 + E\);
\( - {\text{3888 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Do not allow 3889 (given in data booklet).
Allow 3888 (i.e no minus sign).
Award [2] for the correct final answer.
energy required to remove one electron;
from an atom in its gaseous state;
electron removed from a positive ion;
decrease in electron-electron repulsion / increase in nucleus-electron attraction;
MgO;
double ionic charge / both ions carry +2 and –2 charge/greater charge compared to +1 and –1;
\[\begin{array}{*{20}{l}} {\left( {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}} \right)}&{\Delta {H^\Theta } = - {\text{1560;}}} \\ {\left( {{{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + {\text{286;}}} \\ {\left( {{\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + {\text{1411;}}} \\ {\left( {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + {\text{137 (kJ);}}} \end{array}\]
Allow other correct methods.
Award [2] for –137.
Allow ECF for the final marking point.
positive;
increase in number of moles of gas;
at low temperature, \({\Delta {H^\Theta }}\) is positive and \({\Delta G}\) is positive;
at high temperature, factor \({\text{T}}\Delta {S^\Theta }\) predominates and \({\Delta G}\) is negative;
Bonds broken (1C–C, 6C–H, or 1C–C, 2C–H) = 2825/1173;
Bonds made (1C=C, 1H–H, 4C–H) = 2700/1048;
+125 (kJ);
Allow 125 but not –125 (kJ ) for the final mark.
Award [3] for the correct final answer.
bond enthalpy values are average values;
Examiners report
This was the second most popular question and in general candidates demonstrated a good understanding of the Born Haber cycle. Some candidates identified the process A as vaporization instead of atomization.
Most candidates correctly stated the definition of enthalpy change of formation although some omitted to specify the standard conditions.
The majority of candidates correctly calculated the lattice enthalpy value.
The definition of the first ionization energy was stated correctly by most candidates but in a few cases the term gaseous state was missing.
The compound with higher lattice enthalpy was correctly identified including the reason.
The majority of candidates manipulated the thermo-chemical equations and calculated the correct answer of +137 kJ although some reversed the sign.
The explanation for why the reaction was non-spontaneous at low temperature but became spontaneous at high temperature was not always precise and deprived many candidates of at least one mark.
The bond enthalpy calculation had the usual mistakes of using the wrong value from the data booklet, bond making minus bond breaking and –125 kJ instead of +125 kJ.
Two students were asked to use information from the Data Booklet to calculate a value for the enthalpy of hydrogenation of ethene to form ethane.
\[{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}\]
John used the average bond enthalpies from Table 10. Marit used the values of enthalpies of combustion from Table 12.
Determine the value for the enthalpy of hydrogenation of ethene using the values for the enthalpies of combustion of ethene, hydrogen and ethane given in Table 12.
Suggest one reason why John’s answer is slightly less accurate than Marit’s answer and calculate the percentage difference.
Markscheme
\(\Delta H = -1411 + ( - 286) - ( - 1560)\) / correct energy cycle drawn;
\( = - 137{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Award [1 max] for incorrect or missing sign.
the actual values for the specific bonds may be different to the average values / the combustion values referred to the specific compounds / OWTTE;
(percentage difference) \( = \frac{{(137 - 125)}}{{137}} \times 100 = 8.76\% \);
Accept \(\frac{{(137 - 125)}}{{125}} \times 100 = 9.60\% \).
Examiners report
In part (b) the formula involving enthalpies of formation was often used instead of a correct enthalpy cycle for the combustion. This caused the majority of candidates to score half marks for these questions.
A few candidates could suggest a reason why one answer was slightly less accurate than the other in part (c). Most could correctly calculate the percentage difference. Surprisingly, several candidates calculated part (a) correctly and part (b) incorrectly, and then determined a percentage difference of more than 200% without seeming to notice that this does not reflect two slightly different answers.
Hydrogen peroxide decomposes according to the equation below.
\({\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\)
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
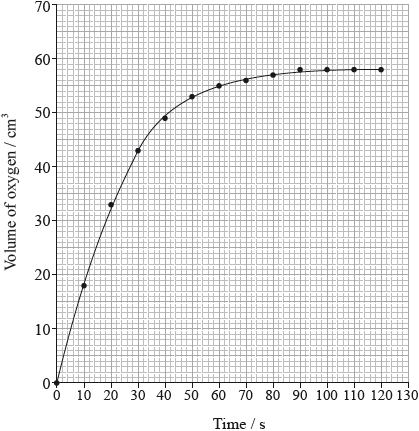
Outline how the initial rate of reaction can be found from the graph.
Explain how and why the rate of reaction changes with time.
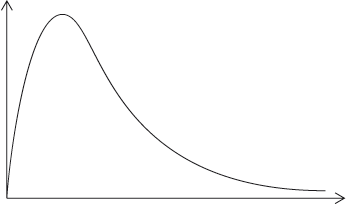
A Maxwell-Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
(i) In some reactions, increasing the concentration of a reactant does not increase the rate of reaction. Describe how this may occur.
(ii) Consider the reaction
\[{\text{2A}} + {\text{B}} \to {\text{C}} + {\text{D}}\]
The reaction is first order with respect to A, and zero order with respect to B. Deduce the rate expression for this reaction.
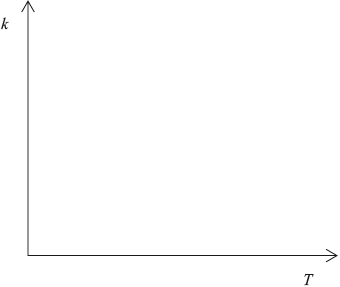
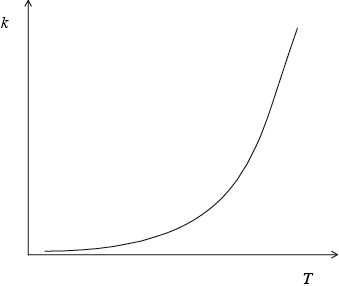
Sketch a graph of rate constant \((k)\) versus temperature.
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = - 57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(i) Define standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Zinc is found in the d-block of the periodic table. Explain why it is not considered a transition metal.
(ii) Explain why \({\text{F}}{{\text{e}}^{3 + }}\) is a more stable ion than \({\text{F}}{{\text{e}}^{2 + }}\) by reference to their electron configurations.
Markscheme
(draw a) tangent to the curve at origin/time = 0/start of reaction;
(calculate) the gradient/slope (of the tangent);
rate decreases (with time);
concentration/number of (reactant) molecules per unit volume decreases (with time);
Do not accept “number of molecules decreases” or “amount of reactant decreases”.
collisions (between reactant molecules/reactant and catalyst) become less frequent;
Do not accept “fewer collisions” without reference to frequency (eg, no. collisions per second).
y-axis: probability / fraction of molecules/particles / probability density
Allow “number of particles/molecules” on y-axis.
and
x-axis: (kinetic) energy;
Accept “speed/velocity” on x-axis.
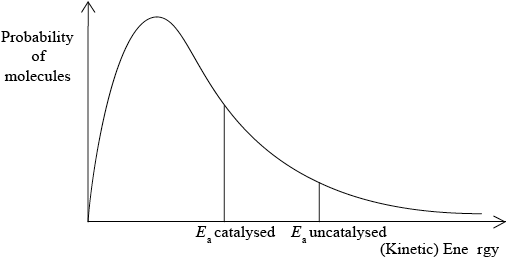
correct relative position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
more/greater proportion of molecules/collisions have the lower/required/catalysed \({E_{\text{a}}}\) (and can react upon collision);
M3 can be scored by stating or shading and annotating the graph.
Accept “a greater number/proportion of successful collisions as catalyst reduces \({E_a}\)”.
(i) reactant not involved in (or before) the slowest/rate-determining step/RDS;
reactant is in (large) excess;
(ii) \({\text{(rate}} = {\text{) }}k{\text{[A]}}\);
Accept rate = k[A]1[B]0.
curve with a positive slope curving upwards;
Do not penalize if curve passes through the origin.
(i) heat transferred/absorbed/released/enthalpy/potential energy change when 1 mol/molar amounts of reactant(s) react (to form products) / OWTTE;
under standard conditions / at a pressure 100 kPa/101.3 kPa/1 atm and temperature 298 K/25 °C;
Award [2] for difference between standard enthalpies of products and standard enthalpies of reactants / \({H^\Theta }\) (products) – \({H^\Theta }\) (reactants).
Award [2] for difference between standard enthalpies of formation of products and standard enthalpies of formation of reactants / \(\Sigma \Delta H_f^\Theta \) (products) – \(\Sigma \Delta H_f^\Theta \) (reactants).
(ii) \((1.00 \times 0.0500 = ){\text{ }}0.0500{\text{ (mol)}}\);
\((0.0500 \times 57.9 = ){\text{ }}2.90{\text{ (kJ)}}\);
Ignore any negative sign.
Award [2] for correct final answer.
Award [1 max] for 2900 J.
(iii) \(\left( {\frac{{2.50}}{{40.00}} = } \right){\text{ }}0.0625{\text{ (mol NaOH)}}\);
\(0.0500 \times 4.18 \times 13.3 = 2.78{\text{ (kJ)}}/50.0 \times 4.18 \times 13.3 = 2780{\text{ (J)}}\);
\(\left( {\frac{{2.78}}{{0.0625}}} \right) = - 44.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Negative sign is necessary for M3.
Award M2 and M3 if is used to obtain an enthalpy change of –46.7 (kJ mol–1).
(iv) \( - 44.5 - 57.9\) / correct Hess’s Law cycle (as below) / correct manipulation of equations;

\( - 102.4{\text{ kJ}}\);
Award [2] for correct final answer.
(i) zinc (only) forms the ion \({\text{Z}}{{\text{n}}^{2 + }}\) / has the oxidation state \( + 2\);
Allow forms only one ion / has only one oxidation state.
has full d-subshell/orbitals / does not have a partially filled d-subshell/orbitals (needed to exhibit transition metal properties);
(ii) \({\text{F}}{{\text{e}}^{2 + }}{\text{: 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{6}}}/{\text{[Ar] 3}}{{\text{d}}^{\text{6}}}\) and \({\text{F}}{{\text{e}}^{3 + }}{\text{: 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}/{\text{[Ar] 3}}{{\text{d}}^{\text{5}}}\);
half-full sub-level/3d5 has extra stability;
less repulsion between electrons / electrons singly occupy orbitals / electrons do not have to pair with other electrons;
Accept converse points for Fe2+.
Examiners report
Most candidates related the rate of reaction to the gradient of the curve, but only a few suggested drawing a tangent at \(t = 0\).
Answers were often disappointing and only a few candidates gained full marks.
Candidates often talked about the number of reactant molecules decreasing but neglected to relate this to a lower concentration. Also some candidates still fail to highlight frequency rather than the number of collisions.
Well answered by more than half of the candidates. The labelling of the axes was a challenge for some candidates. The annotation of the diagram with the energy of activation with and without a catalyst was mostly correct, though some weaker students confused it with the effect of temperature and constructed a second curve. Some candidates could not offer an explanation for the third mark.
(i) Only a few candidates scored this mark. Many candidates stated that a reactant concentration having no effect indicated that the reaction that was zero order in that species, rather than describing the underlying mechanistic reason for the zero order dependence.
(ii) More than half of the candidates could construct a correct rate expression from information about the order of the reactants.
A number of candidates gave a linear relationship, rather than an exponential one, between reaction rate and temperature.
(i) Defining the standard enthalpy change of reaction was not well answered.
(ii) More than half of the candidates calculated the amount of energy released correctly.
(iii) Half of the candidates were able to gain the three marks. Many candidates lost the third mark for not quoting the negative sign for the enthalpy change. Quite a few candidates used a wrong value for the mass of water.
(iv) Many good answers. A Hess’s Law cycle wasn’t often seen. Quite a few candidates scored through ECF from (iii).
(i) Most candidates knew that zinc has a full 3d sub-shell but almost all missed out on the second mark about only having one possible oxidation state in its compounds.
(ii) This was a challenging question for many candidates. A large number of candidates did not give the correct electron configurations for the ions, and only few mentioned the stability of the half-full d-sub-shell. Very few scored the third mark.
Consider the following reaction.
\[{\text{2C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}} + {{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(g)}}\]
The standard enthalpy change of formation for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}}\) at 298 K is \( - 201{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and for \({{\text{H}}_{\text{2}}}{\text{O(g)}}\) is \( - 242{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Using information from Table 11 of the Data Booklet, determine the enthalpy change for this reaction.
The standard entropy for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}}\) at 298 K is \({\text{238 J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) is \({\text{131 J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) and for \({{\text{H}}_{\text{2}}}{\text{O(g)}}\) is \({\text{189 J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\). Using information from Table 11 of the Data Booklet, determine the entropy change for this reaction.
Calculate the standard change in free energy, at 298 K, for the reaction and deduce whether the reaction is spontaneous or non-spontaneous.
Markscheme
\(\Delta H_{{\text{reaction}}}^\Theta = \Sigma \Delta H_{\text{f}}^\Theta {\text{(products)}} - \Sigma \Delta H_{\text{f}}^\Theta {\text{(reactants)}}\)
\( = [(1)( - 85) + (2)( - 242)] - [(2)( - 201)]\);
\( = - 167{\text{ (kJ/kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [1] for (+)167.
\(\Delta S_{{\text{reaction}}}^\Theta = \Sigma {S^\Theta }{\text{(products)}} - \Sigma {S^\Theta }{\text{(reactants)}}\)
\( = [(1)(230) + (2)(189)] - [(2)(238) + (1)(131)]\);
\( = 1{\text{ (J}}\,{{\text{K}}^{ - 1}}{\text{/J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\Delta G_{{\text{reaction}}}^\Theta = (\Delta {H^\Theta } - T\Delta {S^\Theta }) = ( - 167) - (298)(0.001)\);
Award [1] for correct substitution of values.
\( = - 167{\text{ kJ/}} - 167000{\text{ J}}\);
Units needed for mark in (c) only.
Accept –167 kJ\(\,\)mol–1 or –167000 J\(\,\)mol–1.
spontaneous;
Award marks for final correct answers throughout in each of (a), (b) and (c).
Examiners report
In (a) the most common mistakes included: failure to consider the correct amount of moles of products/reactants, incorrect identification of values or wrong use of convention. It also should be noted that the correct units of \(\Delta {H^\Theta }\) here in the answer will be kJ, since \(n\) is used in the equation, as explained in previous subject reports.
Part (b) was another question where the vast majority of candidates scored full marks.
Free energy calculations (c) continues to prove problematic for many candidates. Candidates very often lost the first mark due to wrong use of units. ECF allowed them to score the second. In contrast most candidates showed a clear understanding of the relationship between the sign of \(\Delta {G^\Theta }\) and spontaneity.
Ethanol has many industrial uses.
State an equation for the formation of ethanol from ethene and the necessary reaction conditions.
Equation:
Conditions:
Define the term average bond enthalpy.
Ethanol can be used as a fuel. Determine the enthalpy of combustion of ethanol at 298 K, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), using the values in table 10 of the data booklet, assuming all reactants and products are gaseous.
Students can also measure the enthalpy of combustion of ethanol in the laboratory using calorimetry. Suggest the major source of systematic error in these procedures.
State the equation for the acid-catalysed reaction of ethanol with propanoic acid and state the name of the organic product.
Equation:
Name of the organic product:
A polyester can be formed when ethane-1,2-diol reacts with benzene-1,4-dicarboxylic acid.
Deduce the structure of the repeating unit and state the other product formed.
Repeating unit:
Other product:
State the type of polymerization that occurs.
The standard enthalpy change of combustion, \(\Delta H_{\text{c}}^\Theta \), of propanoic acid is \( - 1527{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine the standard enthalpy change of formation of propanoic acid, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using this information and data from table 12 of the data booklet.
Deduce, giving a reason, the sign of the standard entropy change of the system for the formation of propanoic acid from its elements.
Identify three allotropes of carbon and describe their structures.
Markscheme
Equation:
\({\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH/}}{{\text{C}}_2}{{\text{H}}_4} + {{\text{H}}_2}{\text{O}} \to {{\text{C}}_2}{{\text{H}}_5}{\text{OH}}\);
Conditions:
(concentrated) sulfuric acid/\({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\);
Do not accept dilute sulfuric acid.
Accept phosphoric acid/\({H_3}P{O_4}\) (on pellets of silicon dioxide) (for industrial preparation).
heat / high temperature;
Do not accept warm.
Accept high pressure (for industrial preparation) for M3 only if \({H_3}P{O_4}\) is given for M2.
energy needed to break (1 mol of) a bond in the gaseous state/phase;
(averaged over) similar compounds;
Do not accept “similar bonds” instead of “similar compounds”.
Concept of “similar” is important for M2.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{3}}{{\text{O}}_2} \to {\text{2C}}{{\text{O}}_2} + {\text{3}}{{\text{H}}_2}{\text{O}}\);
Bonds broken:
\(347 + (5 \times 413) + 358 + 464 + (3 \times 498)/4728{\text{ (kJ)}}/{\text{C–C}} + 5{\text{C–H}} + {\text{C–O}} + {\text{O–H}} + {\text{3O=O}}\);
Bonds made:
\((4 \times 746) + (6 \times 464) = 5768{\text{ (kJ)}}/{\text{4C = O}} + {\text{6O–H}}\);
\(\Delta H = (4728 - 5768 = ) - 1040{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\) / bonds broken − bonds formed;
Award [4] for correct final answer.
Award [3] for (+)1040 (\(kJ\,mo{l^{ - 1}}\)).
heat loss (to the surroundings);
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OOCCH2C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}\);
ethyl propanoate;
Do not penalize if equilibrium arrow missing.
Repeating unit:
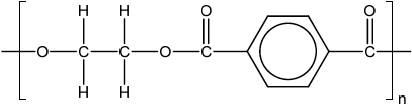 ;
;
Continuation lines must be shown.
Ignore brackets and n.
Accept condensed formulas such as \(C{H_2}\) and \({C_6}{H_4}\).
Other product:
\({{\text{H}}_{\text{2}}}{\text{O}}\)/water;
condensation;
\({\text{3C(s)}} + {\text{3}}{{\text{H}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH(l)}}\);
\(\Delta H_{\text{f}}^\Theta = \sum \Delta H_{\text{c}}^\Theta {\text{ (reactants)}} - \sum {\Delta H_{\text{c}}^\Theta {\text{ (products)}}} \);
Accept any suitable energy cycle.
\(\sum {\Delta H_{\text{c}}^\Theta {\text{ (reactants)}}} = 3 \times ( - 394) + 3 \times ( - 286)/ - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
\((\Delta H_{\text{f}}^\Theta = [3 \times ( - 394) + 3 \times ( - 286)] - ( - 1527) = ) - 513{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
OR
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH(l)}} + {\text{3.5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(g)}}\);
\(\Delta H_{\text{c}}^\Theta = \sum {\Delta H_{\text{f}}^\Theta {\text{ }}(products)} - \sum {\Delta H_{\text{f}}^\Theta {\text{ }}(reactants)} \);
\(\sum {\Delta H_{\text{f}}^\Theta {\text{ (products)}}} = 3 \times ( - 394) + 3 \times ( - 286)/ - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\({\text{(}}\Delta H_{\text{f}}^\Theta = [3 \times ( - 394) + 3 \times ( - 286)] - ( - 1527) = ) - 513{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Ignore state symbols.
Award [4] for correct final answer.
negative;
reduction in the number of gaseous molecules;
Allotropes:
Any three allotropes for [1] from:
diamond
graphite
fullerene
graphene;
Allow (carbon) nanotubes for graphene.
Accept \({C_{{\text{60}}}}\)/\({C_{{\text{70}}}}\)/buckminsterfullerene/bucky balls for fullerene.
Structures:
Any three for [3] from:
Diamond:
tetrahedral arrangement of (carbon) atoms/each carbon bonded to four others / \({\text{s}}{{\text{p}}^{\text{3}}}\) and 3D/covalent network structure;
Graphite:
each carbon bonded to three others (in a trigonal planar arrangement) / \({\text{s}}{{\text{p}}^{\text{2}}}\) and 2D / layers of (carbon) atoms;
Fullerene:
each (carbon) atom bonded to three others (in a trigonal arrangement) / \({\text{s}}{{\text{p}}^{\text{2}}}\) and joined in a ball/cage/sphere/connected hexagons and pentagons;
Accept “trigonal planar” for “each carbon atom bonded to three others” part in M4.
Graphene:
each carbon bonded to three others (in a trigonal arrangement) / \({\text{s}}{{\text{p}}^{\text{2}}}\) and 2D structure;
Examiners report
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
This question is about ethene, C2H4, and ethyne, C2H2.
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
Ethyne reacts with chlorine in a similar way to ethene. Formulate equations for the following reactions.
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHΘ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
Explain, giving two reasons, the difference in the values for (c)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
One possible Lewis structure for benzene is shown.

State one piece of physical evidence that this structure is incorrect.
Markscheme
nickel/Ni «catalyst»
high pressure
OR
heat
Accept these other catalysts: Pt, Pd, Ir, Rh, Co, Ti.
Accept “high temperature” or a stated temperature such as “150 °C”.
[2 marks]
Ignore square brackets and “n”.
Connecting line at end of carbons must be shown.
[1 mark]
ethyne: C2H2 + Cl2 → CHClCHCl
benzene: C6H6 + Cl2 → C6H5Cl + HCl
Accept “C2H2Cl2”.
[2 marks]
ΔHΘ = bonds broken – bonds formed
«ΔHΘ = 3(C≡C) – 6(CC)benzene / 3 \( \times \) 839 – 6 \( \times \) 507 / 2517 – 3042 =» –525 «kJ»
Award [2] for correct final answer.
Award [1 max] for “+525 «kJ»”.
Award [1 max] for:
«ΔHΘ = 3(C≡C) – 3(C–C) – 3(C=C) / 3 \( \times \) 839 – 3 \( \times \) 346 – 3 \( \times \) 614 / 2517 – 2880 =» –363 «kJ».
[2 marks]
ΔHΘ = ΣΔHf (products) – ΣΔHf (reactants)
«ΔHΘ = 49 kJ – 3 \( \times \) 228 kJ =» –635 «kJ»
Award [2] for correct final answer.
Award [1 max] for “+635 «kJ»”.
[2 marks]
ΔHf values are specific to the compound
OR
bond enthalpy values are averages «from many different compounds»
condensation from gas to liquid is exothermic
Accept “benzene is in two different states «one liquid the other gas»” for M2.
[2 marks]
«ΔSΘ = 173 – 3 \( \times \) 201 =» –430 «J K–1»
[1 mark]
T = «25 + 273 =» 298 «K»
ΔGϴ «= –635 kJ – 298 K × (–0.430 kJ K–1)» = –507 kJ
ΔGϴ < 0 AND spontaneous
ΔGϴ < 0 may be inferred from the calculation.
[3 marks]
equal C–C bond «lengths/strengths»
OR
regular hexagon
OR
«all» C–C have bond order of 1.5
OR
«all» C–C intermediate between single and double bonds
Accept “all C–C–C bond angles are equal”.
[1 mark]
Examiners report
Draw the Lewis structures, state the shape and predict the bond angles for the following species.
Consider the following Born-Haber cycle:
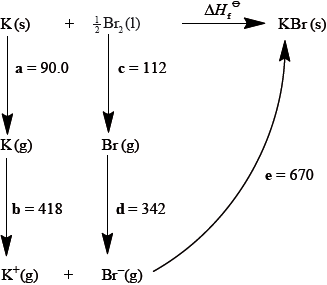
The magnitudes for each of the enthalpy changes (a to e) are given in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) but their signs (+ or –) have been omitted.
\({\text{PC}}{{\text{l}}_{\text{3}}}\)
\({\text{NH}}_2^ - \)
\({\text{Xe}}{{\text{F}}_{\text{4}}}\)
State the names for the enthalpy changes c and d.
Deduce which two of the enthalpy changes a to e have negative signs.
Determine the value for the enthalpy of formation of potassium bromide.
Explain why the quantitative value for the lattice enthalpy of calcium bromide is larger than the value for the lattice enthalpy of potassium bromide.
Compare the formation of a sigma \((\sigma )\) and a pi \((\pi )\) bond between two carbon atoms in a molecule.
Identify how many sigma and pi bonds are present in propene, \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\).
Deduce all the bond angles present in propene.
Explain how the concept of hybridization can be used to explain the bonding in the triple bond present in propyne.
Markscheme
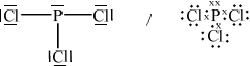 ;
;
trigonal pyramid;
in the range of 100–108°;
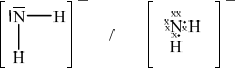 ;
;
Must include minus sign for the mark.
bent/V–shaped;
in the range of 100–106°;
 ;
;
square planar;
90°;
Penalize once only if electron pairs are missed off outer atoms.
c: atomization (enthalpy);
d: electron affinity;
d and e;
\(\Delta {H_{\text{f}}} = 90.0 + 418 + 112 + (-342) + ( - 670)\);
\( = - 392{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
\({\text{C}}{{\text{a}}^{2 + }}\) is smaller than \({{\text{K}}^ + }\) and \({\text{C}}{{\text{a}}^{2 + }}\) has more charge than \({{\text{K}}^ + }\) / \({\text{C}}{{\text{a}}^{2 + }}\) has a greater charge density;
so the attractive forces between the ions are stronger;
Do not accept ‘stronger ionic bonds’
Award [1 max] if reference is made to atoms or molecules instead of ions.
sigma bonds are formed by end on/axial overlap of orbitals with electron density between the two atoms/nuclei;
pi bonds are formed by sideways overlap of parallel p orbitals with electron density above and below internuclear axis/\(\sigma \) bond;
Accept suitably annotated diagrams
8 sigma/\(\sigma \) ;
1 pi/\(\pi \) ;
109°/109.5°;
120°;
sp hybridization;
1 sigma and 2 pi;
sigma bond formed by overlap between the two sp hybrid orbitals (on each of the two carbon atoms) / pi bonds formed by overlap between remaining p orbitals (on each of the two carbon atoms) / diagram showing 2 sp hybrid orbitals and 2 p orbitals;
Examiners report
This question was the most popular of the Section B questions. Part (a) was generally well answered with many candidates drawing clear Lewis structures and applying their knowledge of VSEPR theory well. Common errors included the omission of lone electron pairs on outer atoms, and the omission of a bracket and charge on the ion. Incorrect angular values were common. Some candidates described shapes and bond angles in terms of the ‘parent shape’. Good candidates explained the answers well and scored full marks. Weaker candidates simply wrote two answers; for example, ‘tetrahedral bent’ and could not be awarded marks.
This question was the most popular of the Section B questions. Part (a) was generally well answered with many candidates drawing clear Lewis structures and applying their knowledge of VSEPR theory well. Common errors included the omission of lone electron pairs on outer atoms, and the omission of a bracket and charge on the ion. Incorrect angular values were common. Some candidates described shapes and bond angles in terms of the ‘parent shape’. Good candidates explained the answers well and scored full marks. Weaker candidates simply wrote two answers; for example, ‘tetrahedral bent’ and could not be awarded marks.
This question was the most popular of the Section B questions. Part (a) was generally well answered with many candidates drawing clear Lewis structures and applying their knowledge of VSEPR theory well. Common errors included the omission of lone electron pairs on outer atoms, and the omission of a bracket and charge on the ion. Incorrect angular values were common. Some candidates described shapes and bond angles in terms of the ‘parent shape’. Good candidates explained the answers well and scored full marks. Weaker candidates simply wrote two answers; for example, ‘tetrahedral bent’ and could not be awarded marks.
In part (b) many candidates incorrectly identified the process converting liquid bromine molecules to gaseous bromine atoms as vaporization.
Deducing the enthalpy changes with negative signs proved challenging for many although, with follow through marks credit was earned for the calculation of the enthalpy of formation of potassium bromide.
Some teachers commented on the G2 forms that the energy cycle diagram was strange, however, the stages of the Born-Haber cycle were clearly given and candidates should be familiar with those.
Very few candidates could explain why calcium bromide has a larger lattice enthalpy than potassium bromide. Many referred to atoms instead of ions, and tried to answer this in terms of the electronegativity of the metals.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Carbon monoxide reacts with hydrogen to produce methanol.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\]
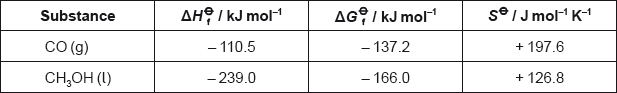
Calculate the standard enthalpy change, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), for the reaction.
Calculate the standard free energy change, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), for the reaction
\({\text{(}}\Delta G_{\text{f}}^\Theta {\text{(}}{{\text{H}}_{\text{2}}}{\text{)}} = {\text{0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\).
Using the values obtained in parts (a) and (b), calculate the standard entropy change, \(\Delta {S^\Theta }\), in \({\text{J mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}}\), for the reaction at 298 K.
Determine the absolute entropy, \({S^\Theta }\), in \({\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}}\), for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) at 298 K.
Markscheme
\(( - 239.0 - [ - 110.5] = ) - 128.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(( - 166.0 - [ - 137.2] = ) - 28.8{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\left( {\Delta {G^\Theta } = - 28.8 = - 128.5 - \left[ {\frac{{298 \times \Delta {S^\Theta }}}{{1000}}} \right]} \right)\)
\((\Delta {S^\Theta } = ) - 335{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}})\);
\(\Delta {S^\Theta } = \sum {S_{products}^\Theta - \sum {S_{reactants}^\Theta } } / - 335 = 126.8 - 197.6 - 2S_{{{\text{H}}_2}}^\Theta \);
\(S_{{{\text{H}}_2}}^\Theta = ( + )132{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}})\);
Award [2] for correct final answer.
Award [1 max] for \(S_{{{\text{H}}_2}}^\Theta = ( + )264{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}})\).
Examiners report
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Trends in physical and chemical properties are useful to chemists.
Cobalt forms the transition metal complex [Co(NH3)4 (H2O)Cl]Br.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
State the shape of the complex ion.
Deduce the charge on the complex ion and the oxidation state of cobalt.
Describe, in terms of acid-base theories, the type of reaction that takes place between the cobalt ion and water to form the complex ion.
Markscheme
Any three of:
Group 1:
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and nucleus for M2.
Accept “weaker metallic bonds” for M2.
Group 17:
number of electrons/surface area/molar mass increase
London/dispersion/van der Waals’/vdw forces increase
Accept “atomic mass” for “molar mass”.
[Max 3 Marks]
«distorted» octahedral
Accept “square bipyramid”.
Charge on complex ion: 1+/+
Oxidation state of cobalt: +2
Lewis «acid-base reaction»
H2O: electron/e– pair donor
OR
Co2+: electron/e– pair acceptor
Examiners report
In December 2010, researchers in Sweden announced the synthesis of N,N–dinitronitramide, \({\text{N(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{3}}}\). They speculated that this compound, more commonly called trinitramide, may have significant potential as an environmentally friendly rocket fuel oxidant.
Methanol reacts with trinitramide to form nitrogen, carbon dioxide and water. Deduce the coefficients required to balance the equation for this reaction.
___ \({\text{N(N}}{{\text{O}}_2}{{\text{)}}_3}{\text{(g)}} + \) ___ \({\text{C}}{{\text{H}}_3}{\text{OH(l)}} \to \) ___ \({{\text{N}}_2}{\text{(g)}} + \) ___ \({\text{C}}{{\text{O}}_2}{\text{(g)}} + \) ___ \({{\text{H}}_2}{\text{O(l)}}\)
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), when one mole of trinitramide decomposes to its elements, using bond enthalpy data from Table 10 of the Data Booklet. Assume that all the N–O bonds in this molecule have a bond enthalpy of \({\text{305 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
The entropy change, \(\Delta S\), for the decomposition of trinitramide has been estimated as \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\). Comment on the sign of \(\Delta S\).
Using \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) as the value for the entropy change, along with your answer to part (c), calculate \(\Delta G\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for this reaction at 300 K. (If you did not obtain an answer for part (c), then use the value \( - 1000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Explain how changing the temperature will affect whether or not the decomposition of trinitramide is spontaneous.
Outline how the length of the N–N bond in trinitramide compares with the N–N bond in nitrogen gas, \({{\text{N}}_{\text{2}}}\).
Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
Predict, with an explanation, the polarity of the trinitramide molecule.
Markscheme
\(\underline {{\text{ (1) }}} {\text{N(N}}{{\text{O}}_2}{{\text{)}}_3}{\text{(g)}} + \underline {{\text{ 2 }}} {\text{C}}{{\text{H}}_3}{\text{OH(l)}} \to \underline {{\text{ 2 }}} {{\text{N}}_2}{\text{(g)}} + \underline {{\text{ 2 }}} {\text{C}}{{\text{O}}_2}{\text{(g)}} + \underline {{\text{ 4 }}} {{\text{H}}_2}{\text{O(l)}}\);
bonds broken: \((6 \times 305) + (3 \times 158) = 1830 + 474 = 2304{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
bonds made: \((2 \times 945) + (3 \times 498) = 1890 + 1494 = 3384{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
enthalpy change: \(2304 - 3384 = - 1080{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Award [2 max] for +1080 (kJ mol–1).
Accept –234 kJ mol–1 which arise from students assuming that 305 kJ mol–1 refers to the strength of a single N–O bond. Students may then take N=O from the data book value (587 kJ mol–1).
bonds broken: (3 \( \times \) 305) + (3 \( \times \) 587) + (3 \( \times \) 158) = 915 + 1761 + 474 = 3150 (kJ mol–1)
bonds made: (2 \( \times \) 945) + (3 \( \times \) 498) = 1890 + 1494 = 3384(kJ mol–1)
enthalpy change: 3150 – 3384 = –234(kJ mol–1).
Award [2 max] for correct calculation of the enthalpy change of reaction for the equation in part (a), which gives –2160 (kJ mol–1).
Award [1] if the final answer is not –2160 but the candidate has correctly calculated the bonds broken in trinitramide as 2304 (kJ mol–1).
increase in the number of moles of gas;
gases have a greater entropy/degree of randomness (than liquids or solids);
Award [1 max] for answers stating that positive value indicates an increase in disorder/randomness.
\(\Delta G = \Delta H - T \times \Delta S\);
\( = - 1080 - 300 \times \frac{{700}}{{1000}}\);
\( - 1290{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Award [2 max] for incorrect conversions of units.
If no answer to part (c), using \(\Delta H\) = –1000 kJ mol–1, gives –1020 (kJ mol–1).
no change in spontaneity / temp has no effect on spontaneity / spontaneous at all temperatures;
\(\Delta G\) negative at all temperatures / exothermic/\(\Delta H\) negative and involves an increase in entropy/\(\Delta S\) positive;
(N–N bond in) trinitramide is longer/nitrogen (gas) is shorter / 0.145 nm in trinitramide versus 0.110 nm in nitrogen;
trinitramide has single (N–N) bond and nitrogen (gas) has triple bond;
106° – 108°;
Accept < 109°.
Any two for [2 max].
4 (negative) charge centres/electron pairs/electron domains around central nitrogen;
central nitrogen has a lone/non-bonding pair;
lone/non-bonding pairs repel more than bonding pairs;
molecule will be (trigonal/triangular) pyramidal;
(negative) charge centres/electron pairs/electron domains will be tetrahedrally arranged/orientated/ have tetrahedral geometry;
Do not apply ECF.
polar;
net dipole moment present in molecule / unsymmetrical distribution of charge / polar bonds do not cancel out / centre of negatively charged oxygen atoms does not coincide with positively charged nitrogen atom;
Marks may also be awarded for a suitably presented diagram showing net dipole moment.
Do not accept “unsymmetrical molecule”.
Apply ECF from part (h).
Examiners report
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Most students could insert the coefficients to balance the equation provided and many recognized the benign nature of the products formed. Though the structure of trinitramide was not given this did not seem to hinder students in calculating the required enthalpy change. A worryingly high number of students however used bond enthalpies to calculate the enthalpy change in the part (a) equation rather than the much simpler decomposition asked for, so to allow them to gain some credit, the mark scheme was adjusted. The sections relating to entropy and free energy changes were generally well tackled, as was the comparative lengths of the N-N bonds. Predicting the shape and polarity of the trinitramide molecule often proved more difficult, especially explaining the polarity of the molecule. Explanations of the effect of external pressure on boiling point, in terms of vapour pressure, and of the effect of temperature, in terms of kinetic theory, often lacked clarity.
Ethane-1,2-diol, HOCH2CH2OH, reacts with thionyl chloride, SOCl2, according to the reaction below.
HOCH2CH2OH (l) + 2SOCl2 (l) → ClCH2CH2Cl (l) + 2SO2 (g) + 2HCl (g)
Calculate the standard enthalpy change for this reaction using the following data.
Calculate the standard entropy change for this reaction using the following data.
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
Markscheme
ΔHθ = [–165.2 + 2(–296.9) + 2(–92.3)] – [–454.7 + 2(–245.7)]
«ΔHθ = +»2.5 «kJ»
Award [2] for correct final answer.
Award [1] for –2.5 «kJ».
Do not accept ECF for M2 if more than one error in M1.
«ΔSθ = [208.5 + 2(248.1) + 2(186.8)] – [166.9 + 2(278.6)]»
«ΔSθ = +» 354.2 «J K–1 mol–1»
«3 moles of» liquid to «4 moles of» gas
OR
«large» positive ΔS
OR
«large» increase in entropy
TΔS > ΔH «at the reaction temperature»
Examiners report
Soluble acids and bases ionize in water.
A solution containing 0.510 g of an unknown monoprotic acid, HA, was titrated with 0.100 mol dm–3 NaOH(aq). 25.0 cm3 was required to reach the equivalence point.
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
State a technique other than a pH titration that can be used to detect the equivalence point.
Deduce the pKa for this acid.
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
Markscheme
weak AND pH at equivalence greater than 7
OR
weak acid AND forms a buffer region
[1 mark]
calorimetry
OR
measurement of heat/temperature
OR
conductivity measurement
Accept “indicator” but not “universal indicator”.
[1 mark]
«pKa = pH at half-equivalence =» 5.0
[1 mark]
Ka = \({10^{ - 4.35}}/4.46683 \times {10^{ - 5}}\)
[H3O+] = \(\sqrt {4.46683 \times {{10}^{ - 5}} \times 1.60 \times {{10}^{ - 3}}} \,\,\,/\,\,\sqrt {7.1469 \times {{10}^{ - 8}}} \,\,/\,\,2.6734 \times {10^{ - 4}}\) «mol dm–3»
pH = «\( - \log \sqrt {7.1469 \times {{10}^{ - 8}}} = \)» 3.57
Award [3] for correct final answer to two decimal places.
If quadratic equation used, then: [H3O+] = 2.459 × 10–4 «mol dm–3» and pH = 3.61
[3 marks]
Examiners report
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
(i) Calculate ΔHθ, in kJ, for this similar reaction below using \(\Delta H_{\rm{f}}^\theta \) data from section 12 of the data booklet. \(\Delta H_{\rm{f}}^\theta \) of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) \( \rightleftharpoons \) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
Predict the 1HNMR data for ethanedioic acid and ethane-1,2-diol by completing the table.
Markscheme
i
«ΔH = Σ ΔHf products – ΣΔHf reactants = –454.8 kJ mol-1 – 2(–110.5 kJ mol-1) =» –233.8 «kJ»
ii
in (a)(iii) gas is formed and in (b)(i) liquid is formed
OR
products are in different states
OR
conversion of gas to liquid is exothermic
OR
conversion of liquid to gas is endothermic
OR
enthalpy of vapourisation needs to be taken into account
Accept product is «now» a liquid.
Accept answers referring to bond enthalpies being means/averages.
iii
«ΔS is negative because five mols of» gases becomes «one mol of» liquid
OR
increase in complexity of product «compared to reactants»
OR
product more ordered «than reactants»
Accept “fewer moles of gas” but not “fewer molecules”.
iv
ΔS = \(\left( {\frac{{ - 620.1}}{{1000}}} \right)\)«kJ K-1»
ΔG = –233.8 kJ – (298 K \(\left( {\frac{{ - 620.1}}{{1000}}} \right)\) kJ K-1) = –49.0 «kJ»
Award [2] for correct final answer.
Award [1 max] for «+»185 × 103.
If –244.0 kJ used, answer is:
ΔG = –244.0 kJ – (298 K \(\left( {\frac{{ - 620.1}}{{1000}}} \right)\)kJ K-1) = –59.2 «kJ»
Award [2] for correct final answer.
v
increasing T makes ΔG larger/more positive/less negative
OR
–TΔS will increase
Accept “none/no splitting” for singlet.
Examiners report
Phosphine (IUPAC name phosphane) is a hydride of phosphorus, with the formula PH3.
(i) Draw a Lewis (electron dot) structure of phosphine.
(ii) State the hybridization of the phosphorus atom in phosphine.
(iii) Deduce, giving your reason, whether phosphine would act as a Lewis acid, a Lewis base, or neither.
(iv) Outline whether you expect the bonds in phosphine to be polar or non-polar, giving a brief reason.
(v) Phosphine has a much greater molar mass than ammonia. Explain why phosphine has a significantly lower boiling point than ammonia.
(vi) Ammonia acts as a weak Brønsted–Lowry base when dissolved in water.
Outline what is meant by the terms “weak” and “Brønsted–Lowry base”.
Weak:
Brønsted–Lowry base:
Phosphine is usually prepared by heating white phosphorus, one of the allotropes of phosphorus, with concentrated aqueous sodium hydroxide. The equation for the reaction is:
(i) The first reagent is written as P4, not 4P. Describe the difference between P4 and 4P.
(ii) The ion H2PO2− is amphiprotic. Outline what is meant by amphiprotic, giving the formulas of both species it is converted to when it behaves in this manner.
(iii) State the oxidation state of phosphorus in P4 and H2PO2−.
P4:
H2PO2−:
(iv) Oxidation is now defined in terms of change of oxidation number. Explore how earlier definitions of oxidation and reduction may have led to conflicting answers for the conversion of P4 to H2PO2− and the way in which the use of oxidation numbers has resolved this.
2.478 g of white phosphorus was used to make phosphine according to the equation:
(i) Calculate the amount, in mol, of white phosphorus used.
(ii) This phosphorus was reacted with 100.0 cm3 of 5.00 mol dm−3 aqueous sodium hydroxide. Deduce, showing your working, which was the limiting reagent.
(iii) Determine the excess amount, in mol, of the other reagent.
(iv) Determine the volume of phosphine, measured in cm3 at standard temperature and pressure, that was produced.
Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and water.
(i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol phosphine. Calculate the temperature rise using section 1 of the data booklet and the data below.
Standard enthalpy of combustion of phosphine,
Specific heat capacity of air = 1.00Jg−1K−1=1.00kJkg−1K−1
(ii) The oxide formed in the reaction with air contains 43.6% phosphorus by mass. Determine the empirical formula of the oxide, showing your method.
(iii) The molar mass of the oxide is approximately 285 g mol−1. Determine the molecular formula of the oxide.
(iv) State the equation for the reaction of this oxide of phosphorus with water.
(v) Suggest why oxides of phosphorus are not major contributors to acid deposition.
(vi) The levels of sulfur dioxide, a major contributor to acid deposition, can be minimized by either pre-combustion and post-combustion methods. Outline one technique of each method.
Pre-combustion:
Post-combustion:
Markscheme
(i)
Accept structures using dots and/or crosses to indicate bonds and/or lone pair.
(ii)
sp3
Do not allow ECF from a (i).
(iii)
Lewis base AND has a lone pair of electrons «to donate»
(iv)
non-polar AND P and H have the same electronegativity
Accept “similar electronegativities”.
Accept “polar” if there is a reference to a small difference in electronegativity and apply ECF in 1 a (v).
(v)
PH3 has London «dispersion» forces
NH3 forms H-bonds
H-bonds are stronger
OR
London forces are weaker
Accept van der Waals’ forces, dispersion forces and instantaneous dipole – induced dipole forces.
Accept “dipole-dipole forces” as molecule is polar.
H-bonds in NH3 (only) must be mentioned to score [2].
Do not award M2 or M3 if:
• implies covalent bond is the H-bond
• implies covalent bonds break.
Accept “dipole-dipole forces are weaker”.
(vi)
Weak: only partially dissociated/ionized «in dilute aqueous solution»
Brønsted–Lowry base: an acceptor of protons/H+/hydrogen ions
Accept reaction with water is reversible/an equilibrium.
Accept “water is partially dissociated «by the weak base»”.
(i)
P4 is a molecule «comprising 4P atoms» AND 4P is four/separate «P» atoms
OR
P4 represents «4P» atoms bonded together AND 4P represents «4» separate/non-bonded «P» atoms
(ii)
can act as both a «Brønsted–Lowry» acid and a «Brønsted–Lowry» base
OR
can accept and/or donate a hydrogen ion/proton/H+
HPO22– AND H3PO2
(iii)
P4: 0
H2PO2–: +1
Do not accept 1 or 1+ for H2PO2−.
(iv)
oxygen gained, so could be oxidation
hydrogen gained, so could be reduction
OR
negative charge «on product/H2PO2 »/gain of electrons, so could be reduction
oxidation number increases so must be oxidation
Award [1 max] for M1 and M2 if candidate displays knowledge of at least two of these definitions but does not apply them to the reaction.
Do not award M3 for “oxidation number changes”.
(i)
«\(\left\langle {\frac{{2.478}}{{4 \times 30.97}}} \right\rangle \)»= 0.02000 «mol»
(ii)
n(NaOH) = «0.1000 × 5.00 =» 0.500 «mol» AND P4/phosphorus is limiting reagent
Accept n(H2O) = \(\frac{{100}}{{18}}\) = 5.50 AND P4 is limiting reagent.
(iii)
amount in excess «= 0.500 - (3 × 0.02000)» = 0.440 «mol»
(iv)
«22.7 × 1000 × 0.02000» = 454 «cm3»
Accept methods employing pV = nRT, with p as either 100 (454 cm3) or 101.3 kPa (448 cm3). Do not accept answers in dm3.
(i)
temperature rise «=\(\frac{{750 \times 1.00}}{{0.2000 \times 1.00}}\)»=3750«°C/K»
Do not accept −3750.
(ii)
n(P)«=\(\frac{{43.6}}{{30.97}}\)»=1.41 «mol»
n(O) «=\(\frac{{100 - 43.6}}{{16.00}}\)»=3.53 «mol»
«\(\frac{{n\left( {\rm{O}} \right)}}{{n\left( {\rm{P}} \right)}}\)=\(\frac{{3.53}}{{1.41}}\) = 2.50 so empirical formula is» P2O5
Accept other methods where the working is shown.
(iii)
«\(\frac{{285}}{{141.9}}\)=2.00, so molecular formula = 2×P2O5=»P4O10
(iv)
P4O10(s) + 6H2O (l) → 4H3PO4 (aq)
Accept P4O10(s) + 2H2O (l) → 4HPO3 (aq) (initial reaction)
Accept P2O5(s) + 3H2O(l) → 2H3PO4(aq)
Accept equations for P4O6/P2O3 if given in d (iii).
Accept any ionized form of the acids as the products.
(v)
phosphorus not commonly found in fuels
OR
no common pathways for phosphorus oxides to enter the air
OR
amount of phosphorus-containing organic matter undergoing anaerobic decomposition is small
Accept “phosphorus oxides are solids so are not easily distributed in the atmosphere”.
Accept “low levels of phosphorus oxide in the air”.
Do not accept “H3PO4 is a weak acid”.
(vi)
Pre-combustion:
remove sulfur/S/sulfur containing compounds
Post-combustion:
remove it/SO2 by neutralization/reaction with alkali/base
Accept “lime injection fluidised bed combustion” for either, but not both.
Examiners report
The reaction between hydrogen and nitrogen monoxide is thought to proceed by the mechanism shown below.
(i) State the equation for the overall reaction.
(ii) Deduce the rate expression consistent with this mechanism.
(iii) Explain how you would attempt to confirm this rate expression, giving the results you would expect.
(iv) State, giving your reason, whether confirmation of the rate expression would prove that the mechanism given is correct.
(v) Suggest how the rate of this reaction could be measured experimentally.
The enthalpy change for the reaction between nitrogen monoxide and hydrogen is −664 kJ and its activation energy is 63 kJ.
(i) Sketch the potential energy profile for the overall reaction, using the axes given, indicating both the enthalpy of reaction and activation energy.
(ii) This reaction is normally carried out using a catalyst. Draw a dotted line labelled “Catalysed” on the diagram above to indicate the effect of the catalyst.
(iii) Sketch and label a second Maxwell–Boltzmann energy distribution curve representing the same system but at a higher temperature, Thigher.
(iv) Explain why an increase in temperature increases the rate of this reaction.
One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen monoxide, N2O. This can be represented by the resonance structures below:
(i) Analyse the bonding in dinitrogen monoxide in terms of σ-bonds and Δ-bonds.
(ii) State what is meant by resonance.
Markscheme
(i)
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)
(ii)
rate = k [NO]2[H2]
(iii)
test the effect «on the reaction rate» of varying each concentration «independently»
OR
test the effect of varying [NO] «on rate», whilst keeping [H2] constant AND test effect of varying [H2] «on rate», whilst keeping [NO] constant
rate proportional to [NO]2
OR
doubling [NO] quadruples rate
rate proportional to [H2]
OR
doubling [H2] doubles rate
Remember to refer back to a (ii) for ECF.
If only one species in rate expression, third mark can be awarded for zero order discussion.
(iv)
no AND different mechanisms could give the same rate expression
OR
no AND mechanisms can only be disproved
OR
no AND just suggest it is consistent with the mechanism given
OR
no AND does not give information about what occurs after RDS
(v)
change of pressure «at constant volume and temperature» with time
OR
change of volume «at constant pressure and temperature» with time
Accept other methods where rate can be monitored with time
(i)
products lower than reactants AND enthalpy of reaction correctly marked and labelled with name or value
activation energy correctly marked and labelled with name or value
Accept other clear ways of indicating energy/ enthalpy changes.
(ii)
lower dotted curve, between same reactants and products levels, labelled “Catalysed”
(iii)
second curve at a higher temperature is correctly drawn (maximum lower and to right of original)
(iv)
greater proportion of molecules have E ≥ Ea or E > Ea
OR
greater area under curve to the right of the Ea
greater frequency of collisions «between molecules»
OR
more collisions per unit time/second
Do not accept just particles have greater kinetic energy.
Do not accept just “more collisions”.
(i)
ALTERNATIVE 1:
σ-bond from N to N AND from N to O
π-bond from N to N
delocalized π-bond/π-electrons «extending over the oxygen and both nitrogens»
ALTERNATIVE 2:
both have 2 σ-bonds «from N to N and from N to O» AND π-bond from N to N
one structure has second π-bond from N to N and the other has π-bond from N to O
delocalized π-bond/π-electrons
Award [1 max] if candidate has identified both/either structure having 2 σ-bonds and 2 π-bonds
(ii)
more than one possible position for a multiple/π-/pi- bond
Accept “more than one possible Lewis structure”.
Accept reference to delocalisation if M3 not awarded in c (i).
Accept reference to fractional bond orders.
Examiners report
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) At exactly 600°C the value of the equilibrium constant is 0.200. Calculate the standard Gibbs free energy change, , for the reaction, in kJ, using sections 1 and 2 of the data booklet. State your answer to three significant figures.
(iii) The standard enthalpy change of formation of phosgene, \(\Delta H_f^\Theta \), is −220.1kJmol−1. Determine the standard enthalpy change, \(\Delta H_{}^\Theta \), for the forward reaction of the equilibrium, in kJ, using section 12 of the data booklet.
(iv) Calculate the standard entropy change, \(\Delta S_{}^\Theta \), in JK−1, for the forward reaction at 25°C, using your answers to (a) (ii) and (a) (iii). (If you did not obtain an answer to (a) (ii) and/or (a) (iii) use values of +20.0 kJ and −120.0 kJ respectively, although these are not the correct answers.)
One important industrial use of phosgene is the production of polyurethanes. Phosgene is reacted with diamine X, derived from phenylamine.
(i) Classify diamine X as a primary, secondary or tertiary amine.
(ii) Phenylamine, C6H5NH2, is produced by the reduction of nitrobenzene, C6H5NO2. Suggest how this conversion can be carried out.
(iii) Nitrobenzene can be obtained by nitrating benzene using a mixture of concentrated nitric and sulfuric acids. Formulate the equation for the equilibrium established when these two acids are mixed.
(iv) Deduce the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
The other monomer used in the production of polyurethane is compound Z shown below.
(i) State the name, applying IUPAC rules, of compound Z and the class of compounds to which it belongs.
Name:
Class:
(ii) Deduce the number of signals you would expect to find in the 1H NMR spectrum of compound Z, giving your reasons.
The mass spectrum and infrared (IR) spectrum of compound Z are shown below:
Mass spectrum
IR spectrum
(iii) Identify the species causing the large peak at m/z=31 in the mass spectrum.
(iv) Identify the bond that produces the peak labelled Q on the IR spectrum, using section 26 of the data booklet.
Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of phenylamine at 298K using section 21 of the data booklet.
Markscheme
(i)
\( \ll {K_{\rm{C}}}{\rm{ = }} \gg \frac{{\left[ {{\rm{COC}}{{\rm{l}}_{\rm{2}}}} \right]}}{{\left[ {{\rm{CO}}} \right]\left[ {{\rm{C}}{{\rm{l}}_2}} \right]}}\)
(ii)
T«= 600 + 273» = 873K
ΔGΘ = −8.31 × 873 × ln (0.200)
OR
ΔGΘ = « + » 11676 «J»
ΔGΘ = « + » 11.7 «kJ»
Accept 11.5 to 12.0.
Award final mark only if correct sig fig.
Award [3] for correct final answer.
(iii)
ΔHΘ = −220.1 − (−110.5)
ΔHΘ = −109.6 «kJ»
Award [2] for correct final answer.
Award [1] for −330.6, or +109.6 «kJ».
(iv)
ΔGΘ= −109.6 − (298 × ΔSΘ) = +11.7 «kJ»
ΔSΘ«\(\frac{{\left( {11.7 + 109.6} \right) \times {{10}^3}}}{{298}}\)»= −407 «JK−1»
Award [2] for correct final answer.
Award [2] for −470 «JK−1» (result from given values).
Do not penalize wrong value for T if already done in (a)(ii).
Award [1 max] for −0.407 «kJ K−1».
Award [1 max] for −138.9 «J K−1».
(i)
primary
(ii)
ALTERNATIVE 1:
«heat with» tin/Sn AND hydrochloric acid/HCl
aqueous alkali/OH–(aq)
ALTERNATIVE 2:
hydrogen/H2
nickel/Ni «catalyst»
Accept specific equations having correct reactants.
Do not accept LiAlH4 or NaBH4.
Accept Pt or Pd catalyst.
Accept equations having correct reactants.
(iii)
HNO3 + 2H2SO4 NO2+ + 2HSO4− + H3O+
Accept: HNO3 + H2SO4 NO2+ +HSO4− + H2O Accept HNO3 + H2SO4
H2NO3+ + HSO4− .
Accept equivalent two step reactions in which sulfuric acid first behaves as a strong acid and protonates the nitric acid, before behaving as a dehydrating agent removing water from it.
(iv)
curly arrow going from benzene ring to N of +NO2/NO2+
carbocation with correct formula and positive charge on ring
curly arrow going from C–H bond to benzene ring of cation
formation of organic product nitrobenzene AND H+
Accept mechanism with corresponding Kekulé structures.
Do not accept a circle in M2 or M3. Accept first arrow starting either inside the circle or on the circle.
M2 may be awarded from correct diagram for M3.
M4: Accept C6H5NO2 + H2SO4 if HSO4− used in M3.
(i)
Name: ethane-1,2-diol
Class: alcohol«s»
Accept ethan-1,2-diol / 1,2-ethanediol.
Do not accept “diol” for Class.
(ii)
two AND two hydrogen environments in the molecule
OR
two AND both CH2 and OH present
(iii)
+CH2OH
Accept CH3O+.
Accept [•CH2OH]+ and [•CH3O]+.
Do not accept answers in which the charge is missing.
(iv)
oxygen-hydrogen «bond»/O–H «in hydroxyl»
\({K_{\rm{b}}} \approx \frac{{{{\left[ {{\rm{O}}{{\rm{H}}^ - }} \right]}^2}}}{{\left[ {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}} \right]}} = {10^{ - 9.13}}/7.413 \times {10^{ - 10}}\)
\(\left[ {{\rm{O}}{{\rm{H}}^ - }} \right] = \sqrt {0.0100 \times {{10}^{ - 9.13}}} = 2.72 \times {10^{ - 6}}\)
\(\left[ {{{\rm{H}}^ + }} \right] = \frac{{1 \times {{10}^{ - 14}}}}{{2.72 \times {{10}^{ - 6}}}} = 3.67 \times {10^{ - 9}}\)
OR
pOH = 5.57
pH = −log [H+] = 8.44
Accept other approaches to the calculation.
Award [4] for correct final answer.
Accept any answer from 8.4 to 8.5.
Examiners report
A student titrated two acids, hydrochloric acid, HCl (aq) and ethanoic acid, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine their concentration. The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of each acid.
Using the graph, estimate the initial temperature of the solutions.
Determine the maximum temperature reached in each experiment by analysing the graph.
Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
Markscheme
21.4 °C
Accept values in the range of 21.2 to 21.6 °C.
Accept two different values for the two solutions from within range.
HCl: 30.4 «°C»
Accept range 30.2 to 30.6 °C.
CH3COOH: 29.0 «°C»
Accept range 28.8 to 29.2 °C.
CH3COOH is weak acid/partially ionised
energy used to ionize weak acid «before reaction with NaOH can occur»